INDIANAPOLIS – State health officials knew six clinics in Indiana had received a recalled back pain medication linked to an outbreak of fungal meningitis a week before they notified the public, according to documents obtained by The Associated Press.
A series of emails obtained Friday through an AP public records request indicate officials at the Indiana State Department of Health were racing to keep up with an evolving picture as the disease rapidly spread.
"A lot of new information was coming in regularly over the weekend of Sept. 29 and 30 from the Centers for Disease Control and Prevention as the situation began to take shape," Amy Reel, a spokeswoman for the state health agency, told The Associated Press.
"This is a fast breaking and fluid situation," Randy Snyder, acute care director for the agency, told other state health officials in an Oct. 3 email.
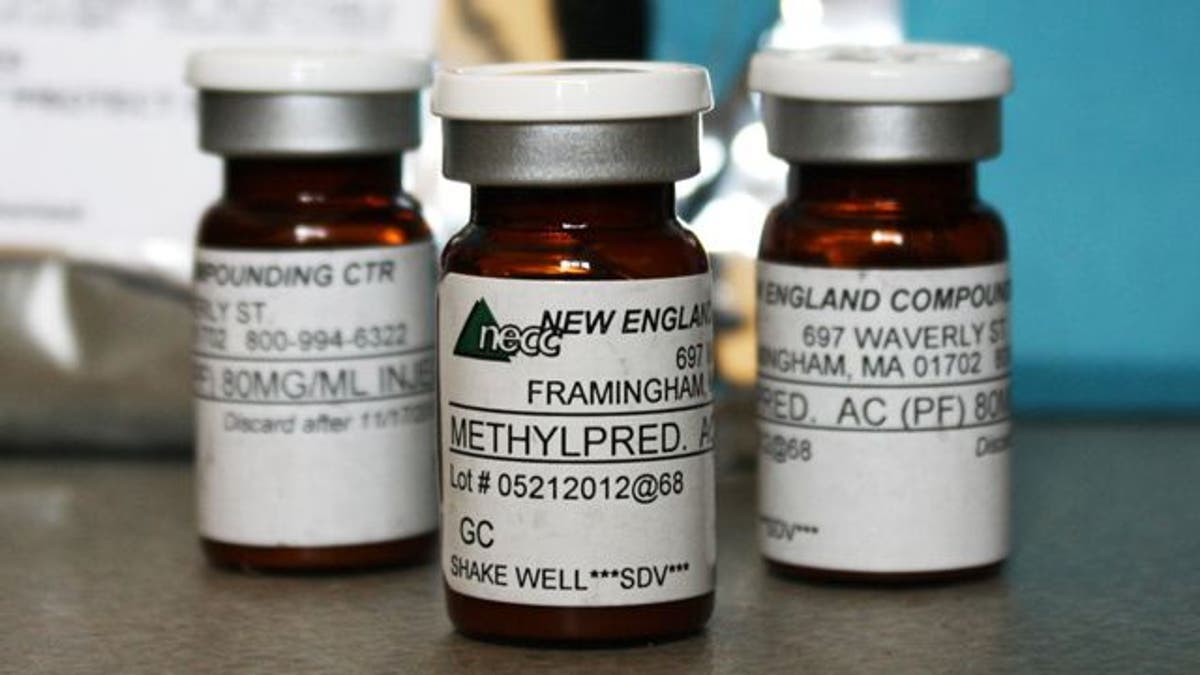
The emails indicate the CDC informed officials at the Indiana health agency on the night of Sept. 28 it was investigating a cluster of fungal meningitis cases in Tennessee and North Carolina that it suspected were connected with injections of a contaminated steroid. An email attachment identified the six Indiana health facilities that were known to have received the suspected medication from a specialty pharmacy in Massachusetts.
The CDC email urged state health agencies to contact clinics that received the tainted steroid and monitor their patients for signs of meningitis symptoms. The Indiana clinics that received the medication were located in Elkhart, Evansville, Fort Wayne, South Bend, Terre Haute and Columbus.
Snyder told officials in an email Sept. 29 that he had received no reports of meningitis cases. That morning, a Saturday, state epidemiologist Pam Pontones sent an email to CDC intelligence officer Rachel Smith saying, "We'll focus on contacting the facilities on the list first."
"We'll contact the patients," Kristi Williams, pharmacy director at Union Hospital in Terre Haute, told state health officials in an email the following Monday. That same day, Oct. 1, Snyder said in an email that the state health department had contacted by phone and email all six of the health facilities that received the tainted lots of medication. The agency also distributed a "script" for the clinics to read from when notifying patients who may have been exposed.
Reel said Indiana and other states didn't receive data defining how to recognize cases that would be considered part of the outbreak from the CDC until Oct. 2, and the state agency immediately alerted physicians.
"It's critical physicians are made aware of signs and symptoms prior to any public announcement so they can be prepared to discuss those signs and symptoms and appropriately treat patients," Reel said.
As of 6:30 a.m. Oct. 4, health officials weren't aware of any meningitis cases in Indiana, according to the documents. But by shortly after noon, they knew that one case had been confirmed and two more were suspected.
"One case has been identified in Indiana, and we probably have at least two more," Pontones said in an email.
That same day, the state health agency informed the clinics that the state epidemiologist would be contacting them for a list of the patients who had received the tainted injections, and to find out how many patients the clinics had already contacted.
In the same email, Snyder told the clinics that the agency intended to announce the outbreak to the public and planned to identify the facilities that had received the contaminated steroid.
Reel said the state health agencies coordinated the release of information with a CDC press conference on Oct. 4, the same day the first Indiana case was confirmed.
As of Sunday, the CDC meningitis website was reporting 44 cases and three deaths connected with Indiana.
The tainted steroids have been traced to the New England Compounding Center of Framingham, Mass., which has since recalled the contaminated lots and suspended production.








































