Fox News Flash top headlines for March 18
Fox News Flash top headlines are here. Check out what's clicking on Foxnews.com.
Moderna asked the Food and Drug Administration (FDA) on Thursday to authorize a fourth shot of its COVID-19 vaccine.
The biotech company wrote in a release that it had requested the agency amend the emergency use authorization (EUA) to allow for the additional booster in adults ages 18 years and older who have received an initial booster of any of the authorized or approved vaccines.
"The request to include adults over 18 years of age was made to provide flexibility for the U.S. Centers for Disease Control and Prevention (CDC) and healthcare providers to determine the appropriate use of an additional booster dose of mRNA-1273, including for those at higher risk of COVID-19 due to age or comorbidities," the vaccine-maker said. "This submission is based in part on recently published data generated in the United States and Israel following the emergence of omicron."
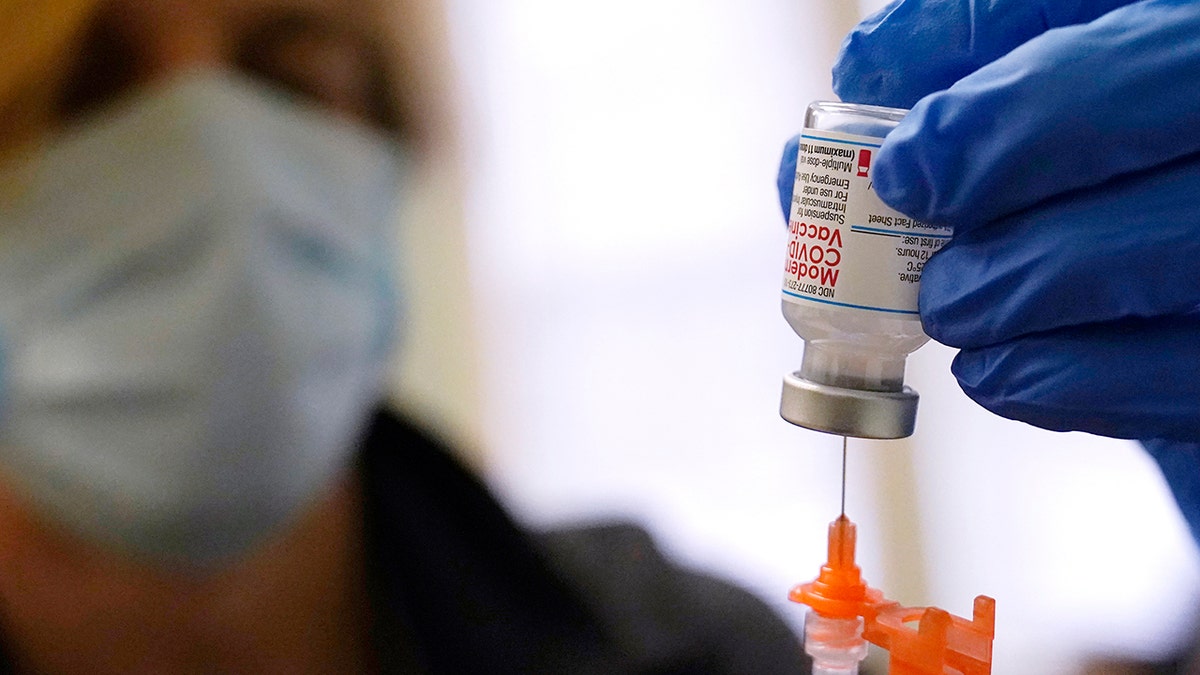
Pharmacist Kenni Clark prepares a booster dose of the Moderna COVID-19 vaccine during a vaccination clinic at City of Lawrence's "The Center," which serves seniors, families and the community, Wednesday, Dec. 29, 2021, in Lawrence, Mass. (AP Photo/Charles Krupa, File)
In November, the FDA amended the EUA for both the Moderna and Pfizer-BioNTech COVID-19 vaccines, authorizing the use of a single booster dose for all individuals 18 years of age and older after completion of primary vaccination with any FDA-authorized or approved COVID-19 vaccine.
The Centers for Disease Control and Prevention (CDC) immediately followed suit, expanding recommendations for booster shots to include all adults ages 18 years and older who received a Pfizer-BioNTech or Moderna vaccine at least six months after their second dose.
WHO SAYS NO EVIDENCE THAT COVID BOOSTERS ARE NEEDED FOR HEALTHY CHILDREN, YOUNG ADULTS
A single booster dose of Moderna's vaccine at the 50-microgram dose level is authorized for emergency use for adults 18 years and older and a third dose of the Moderna vaccine at the 100-microgram dose level is authorized for emergency use in immunocompromised individuals 18 years of age or older who have undergone solid organ transplantation or who are diagnosed with conditions that are considered to have an equivalent level of immunocompromisation.
Moderna noted that clinical trials are ongoing for its omicron-specific booster.
On Tuesday, rivals Pfizer and BioNTech asked U.S. regulators to authorize an additional booster dose of their COVID-19 vaccine for seniors, citing data from Israel that they said suggests older adults would benefit.
Although cases, hospitalizations and deaths have fallen markedly since the winter's omicron variant surge, U.S. officials have been laying the groundwork to deliver additional booster doses.
CLICK HERE TO GET THE FOX NEWS APP
The Biden administration has called on Congress to approve more pandemic funding, sounding the alarm on the potential rise of new variants of concern.
The Associated Press contributed to this report.