Fox News Flash top headlines for September 21
Fox News Flash top headlines are here. Check out what's clicking on Foxnews.com.
A manufacturer is recalling thyroid tablets found to be subpotent and with adverse effects, according to the U.S. Food and Drug Administration (FDA).
Acella Pharmaceuticals is recalling two lots of NP Thyroid, which “may have as low as 87% of the labeled amount of levothyroxine,” or less than the required amount, according to the company announcement posted by the FDA.
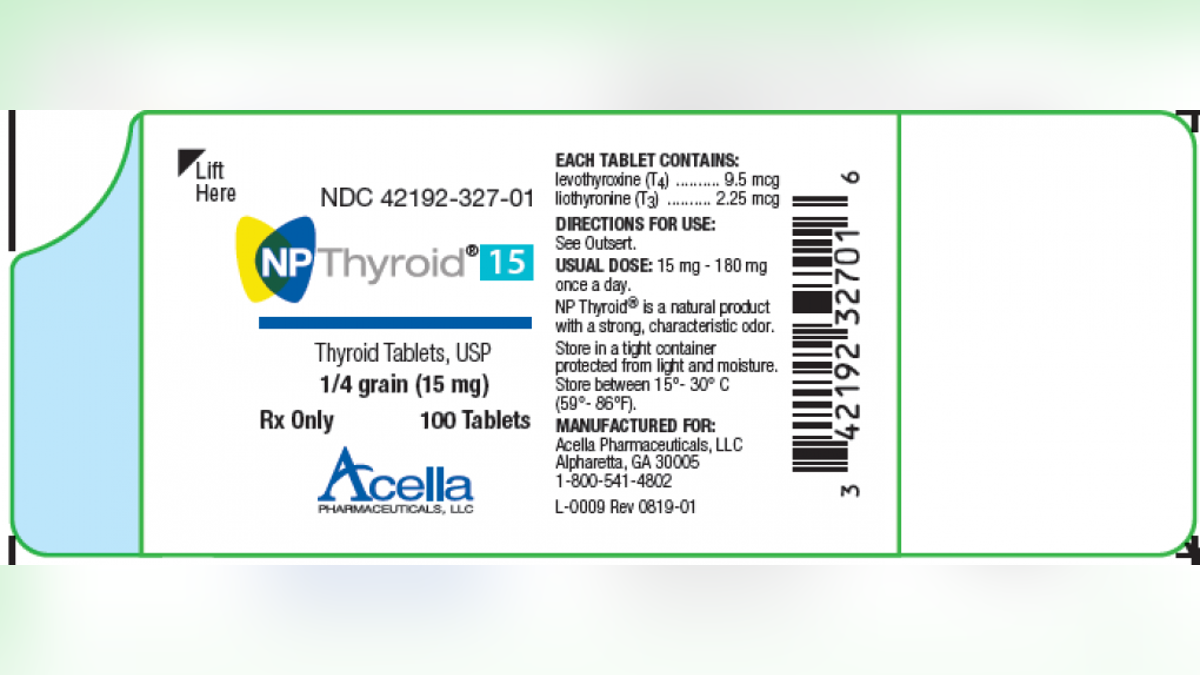
Acella Pharmaceuticals is recalling two lots of NP Thyroid, which “may have as low as 87% of the labeled amount of levothyroxine,” or less than the required amount. (Photo courtesy of the FDA)
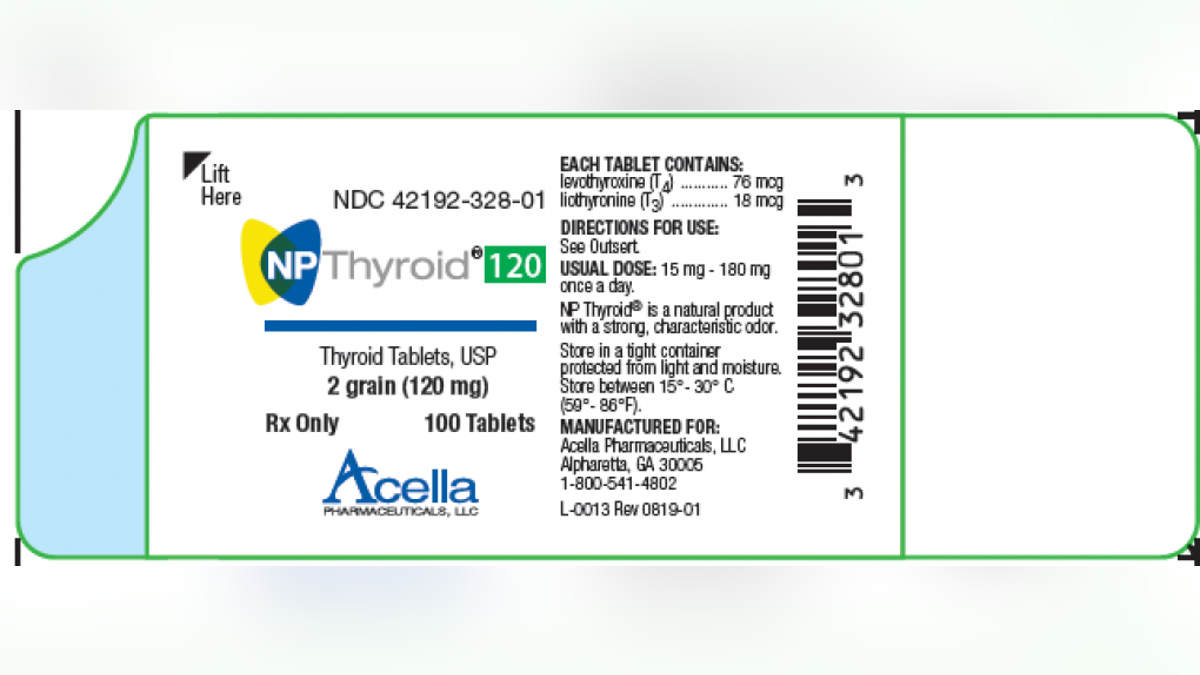
(Photo courtesy of the FDA)
CDC DELETES CORONAVIRUS AIRBORNE TRANSMISSION GUIDANCE, SAYS UPDATE WAS 'DRAFT VERSION'
The products may be too weak to fully treat hypothyroidism (underactive thyroid), and patients could experience fatigue, constipation, and slow heart rate, among other effects, said the announcement.
There was also said to be a “reasonable risk of serious injury” for newborns and pregnant women with hypothyroidism.
"In elderly patients and patients with underlying cardiac disease toxic cardiac manifestations of hyperthyroidism may occur, such as cardiac pain, palpitations or cardiac arrhythmia," the announcement reads.
“To date, Acella has received four reports of adverse events for these lot numbers possibly related to this recall,” it said.
The products under the recall are in 100-count bottles; one lot of 15-mg and a second lot of 120-mg NP Thyroid, Thyroid Tablets. Those taking the medication were advised to contact their healthcare provider before stopping use.
See more information about the recall here.








































