Fox News Flash top headlines for July 16
Fox News Flash top headlines are here. Check out what's clicking on Foxnews.com.
A state-run drug company in China used its own employees as test subjects for COVID-19 vaccine trials even before the government approved the testing for people.
The move -- which raises questions of potential international ethical violations by China's top drug companies -- signals a major leap toward developing a coronavirus vaccine ahead of the rest of the world, and the country's willingness to push boundaries to get there.
“Giving a helping hand in forging the sword of victory,” reads an online post from Beijing-based SinoPharm, with pictures of workers it says helped “pre-test” its vaccine, according to reports by The Associated Press.
RUSSIAN HACKERS BEHIND CYBER ATTACKS ON CORONAVIRUS VACCINE DEVELOPERS: UK INTELLIGENCE
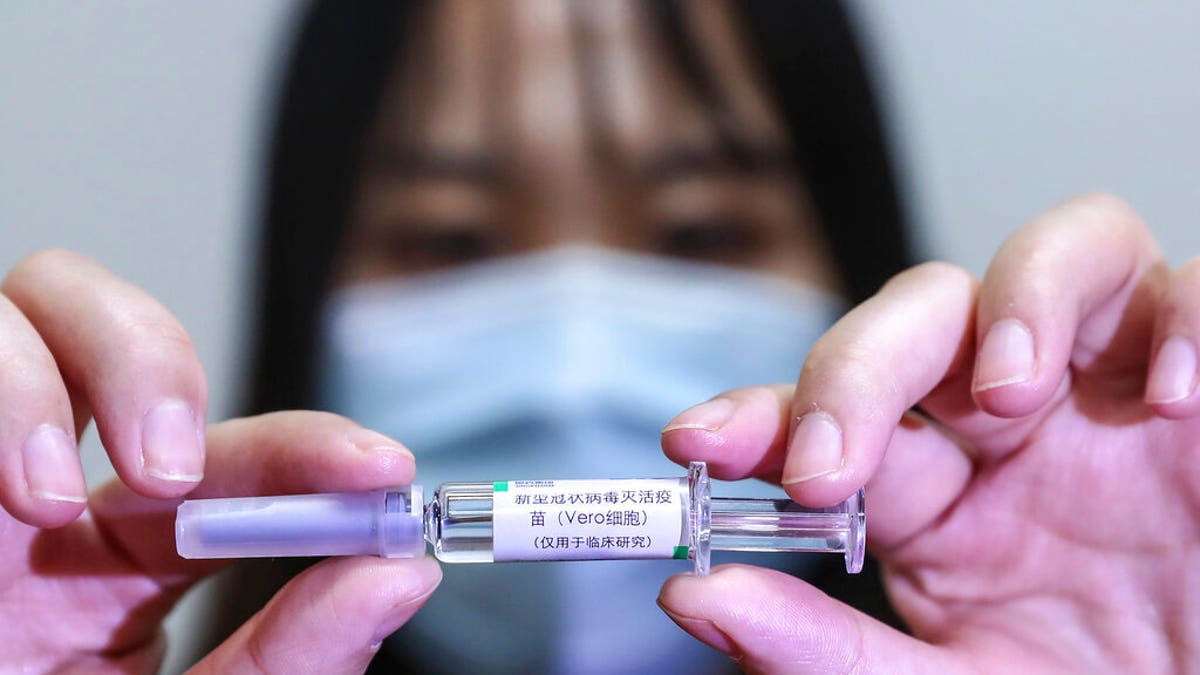
In this April 10, 2020, photo released by Xinhua News Agency, a staff member holds up a sample of a potential COVID-19 vaccine at a production plant of SinoPharm in Beijing. (Zhang Yuwei/Xinhua via AP)
SinoPharm claims that 30 “special volunteers” agreed to be injected with the vaccine even before the company got permission for its initial human study. The company’s post cites a “spirit of sacrifice” and shows seven men in suits and ties — a mix of scientists, businessmen and one Communist Party official with a background in military propaganda.
"Research ethics review and ensuring informed consent for all participants joining a vaccine or therapeutic trial is the ethical bare minimum for any study," Summer McGee, dean of the University of New Haven's School of Health Sciences, told Fox News on Thursday. "China must ensure the trials are sound and not exploiting anyone for the results to be valid and accepted around the world."
Competing against the U.S., Britain and other world players racing to develop a vaccine nearly eight months after coronavirus infected over 13.6 million people across the globe, China's strides appear promising. Eight of nearly two dozen potential vaccines in various stages of human testing worldwide are from China, the most of any country.

In this April 11, 2020, photo released by Xinhua News Agency, a staff member tests samples of a potential COVID-19 vaccine at a production plant of SinoPharm in Beijing. (Zhang Yuwei/Xinhua via AP)
SinoPharm is approved to begin clinical trials in Abu Dhabi in the final phase of vaccine development using up to 15,000 volunteers -- making China and the UAE the first in the world to test an inactivated vaccine -- Middle Eastern city officials announced Thursday, according to Reuters.
China has poured its resources into the inactivated testing technique, which grows a whole virus in the lab and then kills it, using the dead strain of the coronavirus to trigger an immune response in humans, similar to the technique behind the polio vaccine. Western competitors have opted for another, newer and less proven method that targets the “spike” protein that coats the virus.
"The entire world is working at breakneck pace to develop a vaccine, but even in a global pandemic, this is not the time to cut ethical corners," McGee said.

In this April 11, 2020, photo released by Xinhua News Agency, staff members check and clean equipments at a vaccine production plant of SinoPharm in Beijing. (Zhang Yuwei/Xinhua via AP)
CLICK HERE FOR THE FOX NEWS APP
Despite ethical concerns, hundreds of SinoPharm employees, including top executives, have received trials of the vaccine before approvals, Chinese news media have reported.
Subsidiary groups of SinoPharm have already begun building factories capable of generating over 200 million doses of the potential vaccine annually, the company said, according to reports by Fortune.
The Associated Press contributed to this report.