Fox News Flash top headlines for Oct. 24
Fox News Flash top headlines for Oct. 24 are here. Check out what's clicking on Foxnews.com
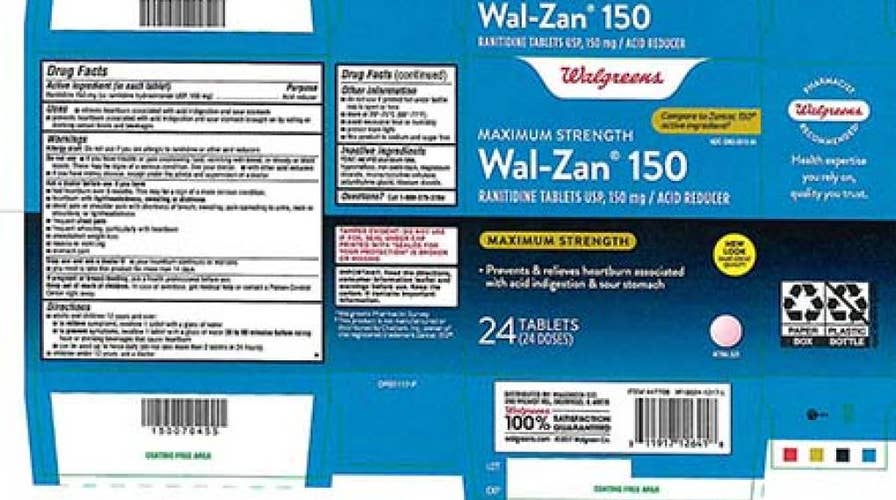
Dr. Reddy’s Laboratories is pulling ranitidine medications sold in the U.S. over concerns about the levels of a cancer-causing ingredient found in samples of the product.
The company said it had not received any reports of adverse reactions due to the products impacted by the recall, but found that the samples were contaminated with N-Nirosodimethylamine (NDMA) above levels established by the FDA.
NDMA is classified as a probable human carcinogen, and the FDA has been testing for the ingredient in ranitidine products from multiple manufacturers to assess the potential impact that it may have on consumers. Ranitidine is available as an over-the-counter medication and is used to treat heartburn, acid indigestion and sour stomach. It’s also available by prescription.
WALMART PULLS ZANTAC FROM SHELVES AMID CANCER CONCERNS
Dr. Reddy’s said the recalled products were available under different names to consumers at Walgreens, Walmart, Sam’s Club, Kroger, Target, CVS and other retailers. A full list of impacted items and UPC numbers can be found on the recall notice posted on the FDA website.
CLICK HERE TO GET THE FOX NEWS APP
The recall comes as major retailers moved to pull Zantac and other generic forms of ranitidine from shelves amid warnings from the FDA about NDMA. The same chemical was linked to dozens of recalls of prescription blood pressure medications over the last year. The health agency said consumers that are currently taking over-the-counter ranitidine could consider using other medicines approved for their condition.