Fox News Flash top headlines for August 19
Fox News Flash top headlines are here. Check out what's clicking on Foxnews.com.
Last week, the U.S. Food and Drug Administration (FDA) was planning to release an emergency use authorization (EUA) for blood plasma as a treatment against COVID-19. But the authorization is now on pause after top experts, namely Dr. Anthony Fauci and Dr. Francis Collins, reportedly stepped in saying the evidence was not strong enough to do so at this time, according to a new report.
Dr. H. Clifford Lane, deputy clinical director at the National Institute of Allergy and Infectious Disease, said the authorization was put "on hold for now" while more data is under review. However, emergency approval is still possible; Lane noted that it "could still be issued in the near future," according to the report from The New York Times.
A spokesperson for the FDA did not immediately return Fox News' request for comment on Wednesday.
MANDATED CORONAVIRUS VACCINATION IN US UNLIKELY, FAUCI SAYS
Fauci, the nation’s top infectious disease expert, and Collins, director of the National Institutes of Health, reportedly urged FDA officials to “hold off” on the authorization, pointing to new data on plasma transfusions from the Mayo Clinic. The study found that convalescent plasma with higher antibody levels transfused to hospitalized COVID-19 patients “significantly reduced mortality” as compared to transfused plasma with low antibody levels. However, Fauci and Collins reportedly believe the study lacks strong enough data to justify an emergency use authorization.
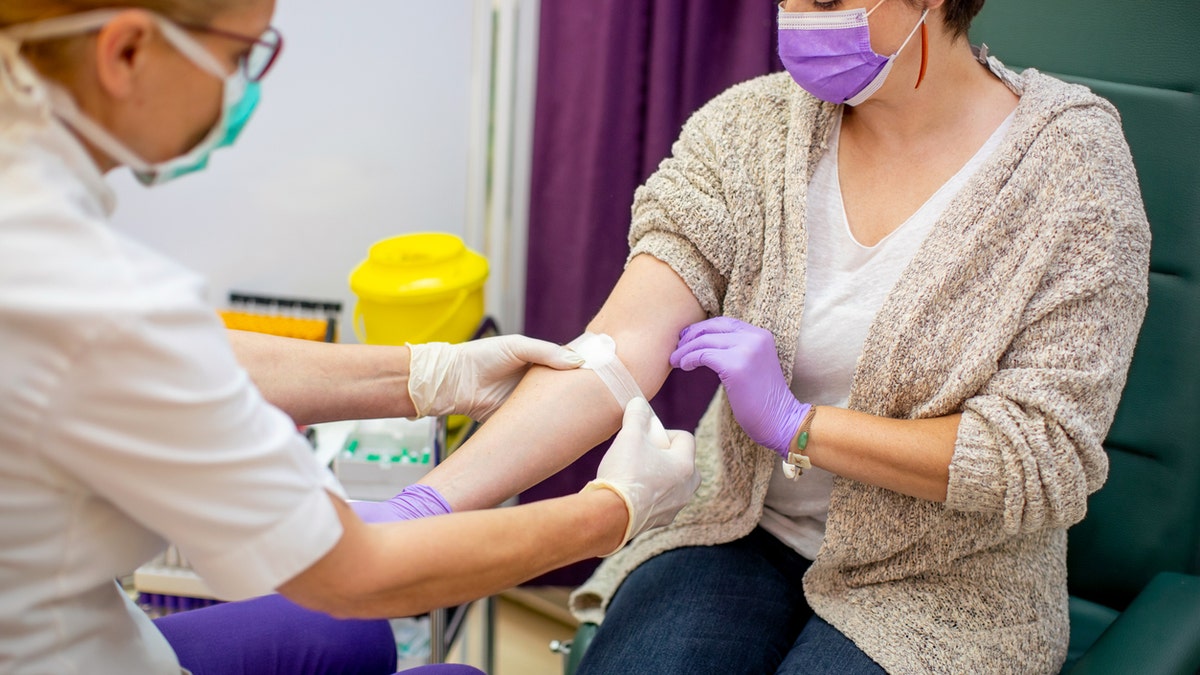
Doctors have hailed plasma transfusions as an effective coronavirus treatment for months. (iStock)
Doctors have hailed plasma transfusions as an effective coronavirus treatment for months, and hospitals and the American Red Cross have been recruiting volunteers to donate plasma to ill coronavirus patients. The idea is that antibodies in the plasma from recovered COVID-19 cases transfused to critically ill COVID-19 patients will help fight or neutralize the disease.
The FDA approves emergency use authorizations during public health emergencies to speed along unapproved medical products (or unapproved uses of such) to treat or prevent serious diseases where there are no other adequate and available alternatives. For instance, on May 1, President Trump announced that the FDA authorized the emergency use of Gilead Science's experimental antiviral drug remdesivir to treat coronavirus patients after early results of a clinical study indicated the drug helps speed recovery.
Also, in mid-June, the FDA revoked the emergency use authorization for chloroquine and hydroxychloroquine donated to the Strategic National Stockpile to treat certain hospitalized coronavirus patients because it determined that the two drugs were unlikely to be effective in treating COVID-19 for the authorized uses in the EUA.
Fox News' Louis Casiano and James Rogers contributed to this report.









































