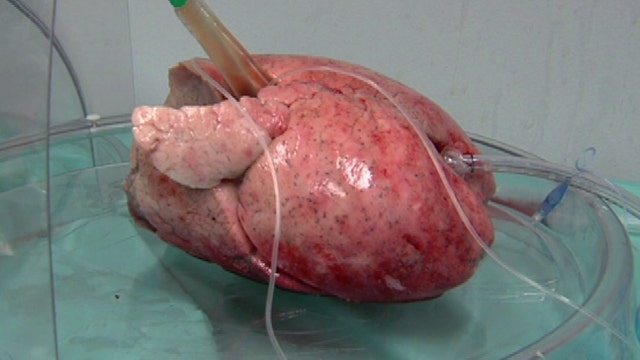
Bringing lungs back to life
Eighty percent of donated lungs can’t be used for transplants because of injury or other problems. Researchers at the Cleveland Clinic are using an experimental process to help revive those lungs that would otherwise be deemed unusable
The U.S. Food and Drug Administration (FDA) has approved a device for preserving donated lungs— which may result in more lung transplants.
The device will allow donated lungs that do not initially meet standard criteria for lung transplantation to be saved for observation and evaluation in the hopes that they may be transplantable later.
Every year, approximately 1,700 lung transplants are performed in the U.S.— a number that could be higher, but only a very small amount of donor organs are suitable for transplant.
“Right now, we are only able to use about 20 percent or so of lungs from those donors,” said Dr. Kenneth McCurry, program and surgical director of heart and lung transplantation at the Cleveland Clinic. “Obviously, that limits how many patients we can transplant, and there are still patients who die waiting to get organs on the waiting list.”
The XVIVO Perfusion System (XPS) with STEEN Solution will allow doctors additional time to determine if a donated lung meets the standard criteria for transplantation. XPS works by warming the donor lungs to near normal body temperature and continually flushing the lung tissue with a sterile fluid solution, called STEEN Solution, to preserve the lungs and remove waste products. The system also ventilates the lung so transplant teams can observe its airways.
Donor lungs can stay in XPS for up to four hours. If they pass evaluation and meet certain functionality criteria, they are transplanted into a recipient.
The Cleveland Clinic has been using the experimental treatment since 2012.
“Of all the organs that we have taken to our lab and used on our machines or resuscitated on our machines, about 50 to 60 percent of those, after a few hours, have improved to the point that we would think they can be transplanted,” McCurry said.