Amgen Inc said its experimental and novel treatment for advanced melanoma met the main goal of a late-stage clinical trial by demonstrating a durable response in significantly more patients than a comparison treatment.
The drug known as talimogene laherparepvec induced a durable response rate (DRR) - defined as a complete or partial tumor shrinkage lasting for at least six months - in 16 percent of patients, according to preliminary results.
While that is a relatively small percentage, that was considered to be statistically significant compared with a DRR of just 2 percent for the comparison treatment, subcutaneous granulocyte-macrophage colony-stimulating factor (GM-CSF), in the difficult to treat disease.
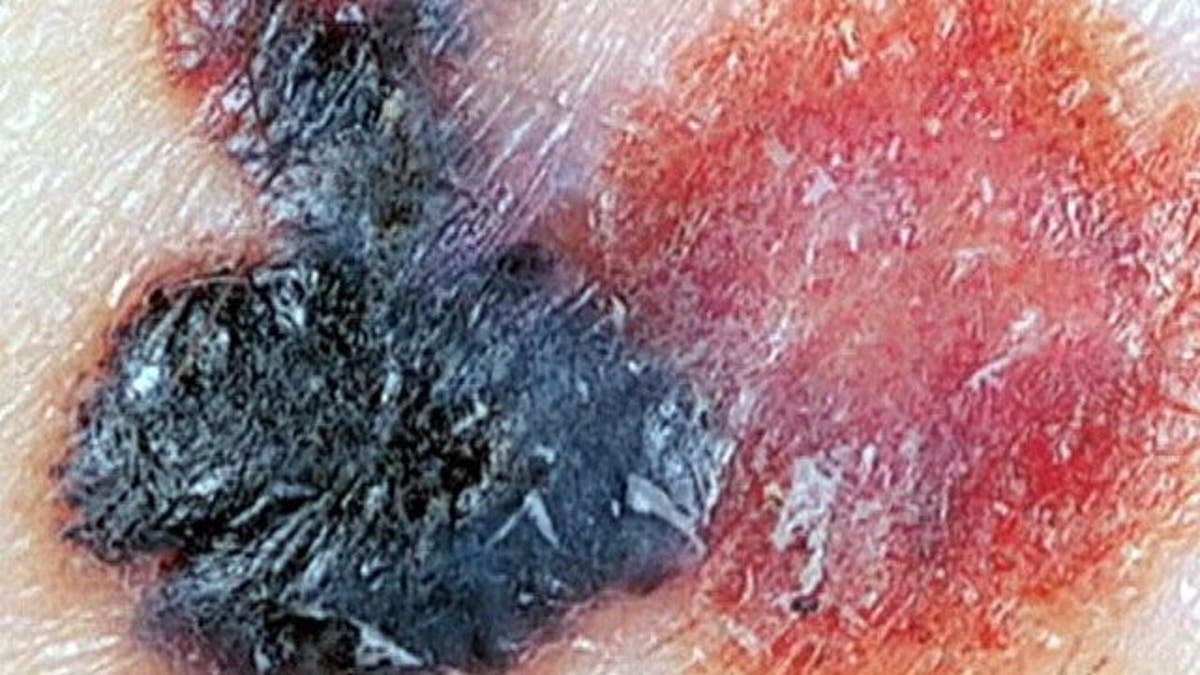
Melanoma is the deadliest of skin cancers, accounting for some 75 percent of all skin cancer deaths.
The company did not yet have data on overall survival from the 400-patient study, but said interim analysis showed a trend toward an overall survival benefit favoring the Amgen drug.
Talimogene laherparepvec, which is injected directly into the tumor, has a unique dual mechanism of action. It is designed to replicate inside the tumor until membranes of the cancer cells rupture, destroying them. The drug then activates a systemic immune response to kill tumor cells throughout the body.
Amgen said it will release more detailed data from the study at a major medical meeting later this year.








































