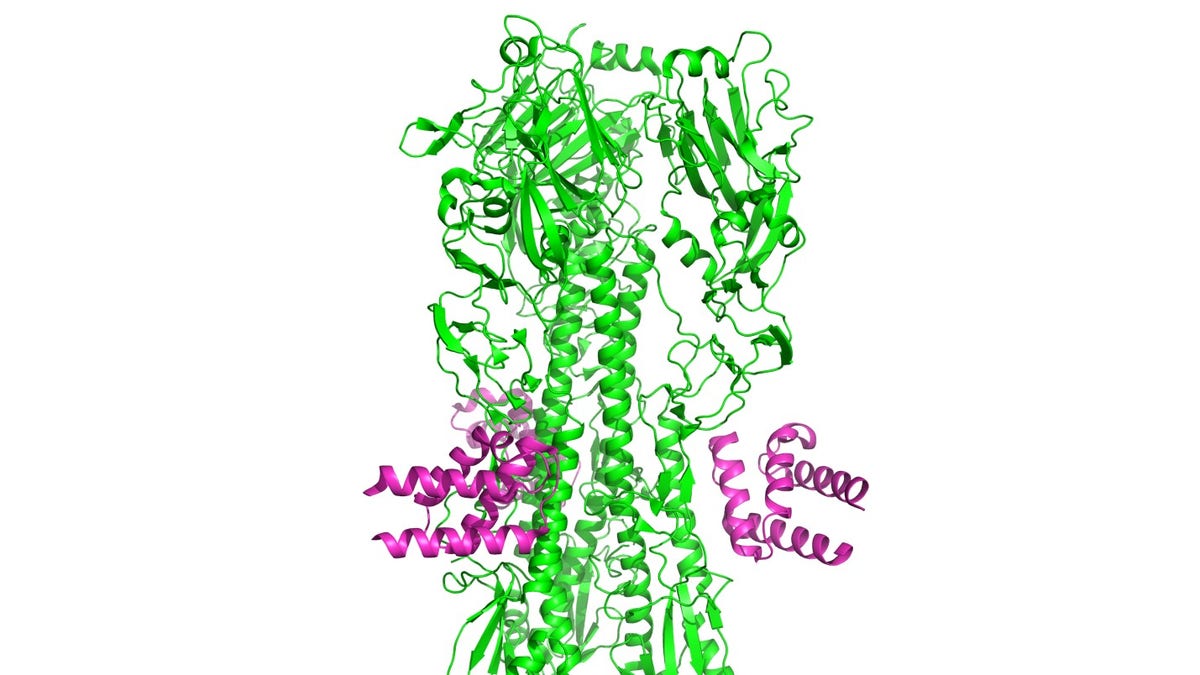
Designed anti-influenza protein HB36.6 (magenta) bound to the hemagglutinin from the H1N1 2009 pandemic virus. (image courtesy: University of Washington)
Researchers at the University of Washington have created an antiviral drug that protects against a range of influenza strains and is more effective than Tamiflu in animal models. The active molecule may also protect those most vulnerable to illness, the immunocompromised.
“It really is important for an antiviral such as this one to be able to neutralize a broad range of infectious strains so if we have an unpredicted pandemic or seasonal strain circulation, it can still afford significant protection,” study author Deborah Fuller, an associate professor in the department of microbiology at the University of Washington in Seattle, told FoxNews.com.
Under magnification, the influenza virus looks somewhat like balls covered with spikes, which are two different proteins, hemagglutinin (HA), and neuraminidase (NA). The proteins consist of an inner stem region— which doesn’t vary much between flu strains— and a highly variable outer blob. Changes in these outer blobs are why flu vaccines differ from season to season— they’re based on researchers’ best prediction of what the coming year’s strains will look like.
This new compound, consisting of the HB36.6 molecule, is designed to interfere with a flu virus in the hemagglutinin (HA) stem, specifically the region that the virus uses to attach to a cell.
“It is not going to stimulate immunity, but really directly interfere with the virus,” Fuller said. “This region is particularly important.”
In animal experiments, HB36.6 was shown to protect flu-infected mice completely. Researchers found that a single dose of HB36.6 provided better protection than 10 doses (twice daily for five days of oseltamivir (Tamiflu); when combined in a low-dose with Tamiflu, all mice survived, indicating a synergistic effect when the two antiviral drugs were combined.
Additionally, the protection granted by this compound does not require the patient to have a fully-functional immune system— which infants, the elderly and immune-compromised lack.
“In a sense, it’s a shift in thinking, saying that we don’t need to recruit immune cells to fight infection, all we need to do is bind the virus in that region,” Fuller said. “Having this protein be able to do this job without depending on a host immune response provides some hope for the potential use in the elderly and immune-compromised.”
While Tamiflu also does not require an immune response, it targets a different part of the virus that has mutation potential and could potentially escape the drug.
“There’s been a lot of variable reports that Tamiflu is not very effective; it might be the emergence of drug resistance, but it might be strains targeting other regions not as effectively,” Fuller said.
Researchers believe the HB36.6’s successful protection may be because it’s administered inter-nasally, putting the drug right in the lungs.
“It provides a barrier right at the initial site of exposure to influenza; we think that’s why it’s really potent,” Fuller said. “That coupled with the fact that [HB36.6] is going after this highly-conserved region on the virus that it uses to infect.”
Inter-nasal injections also potentially allow patients to self-administer the antiviral drug, Fuller added.
The drug does was not found to cause any inflammation, which suggests it wouldn’t cause side effects, unlike existing drugs, researchers noted. HB36.6, which was developed using computational prediction, was also effective when tested as a protective treatment before infection, suggesting potential prophylactic use.
Researchers noted that their findings are only the proof of concept. Next, they’ll test in another animal model— ferrets— and aim to move into phase 1 clinical trials for safety.
“There’s a lot of work to be done,” Fuller said. “We know that current antivirals out there do cause development of drug resistant mutations, and we need to address that for our antiviral as well.”
Fuller added that HB36.6 was only shown to neutralize one group of influenza viruses, but did not protect against group 2 viruses. The team aims to design an antiviral to neutralize these strains, with the goal being to have one antiviral covering any type of flu.
“I truly believe it’s feasible,” she said.
The study was published Thursday in PLOS Pathogens.







































