The Massachusetts pharmacy linked to a deadly U.S. meningitis outbreak filed for Chapter 11 bankruptcy and said it would establish a fund to compensate victims.
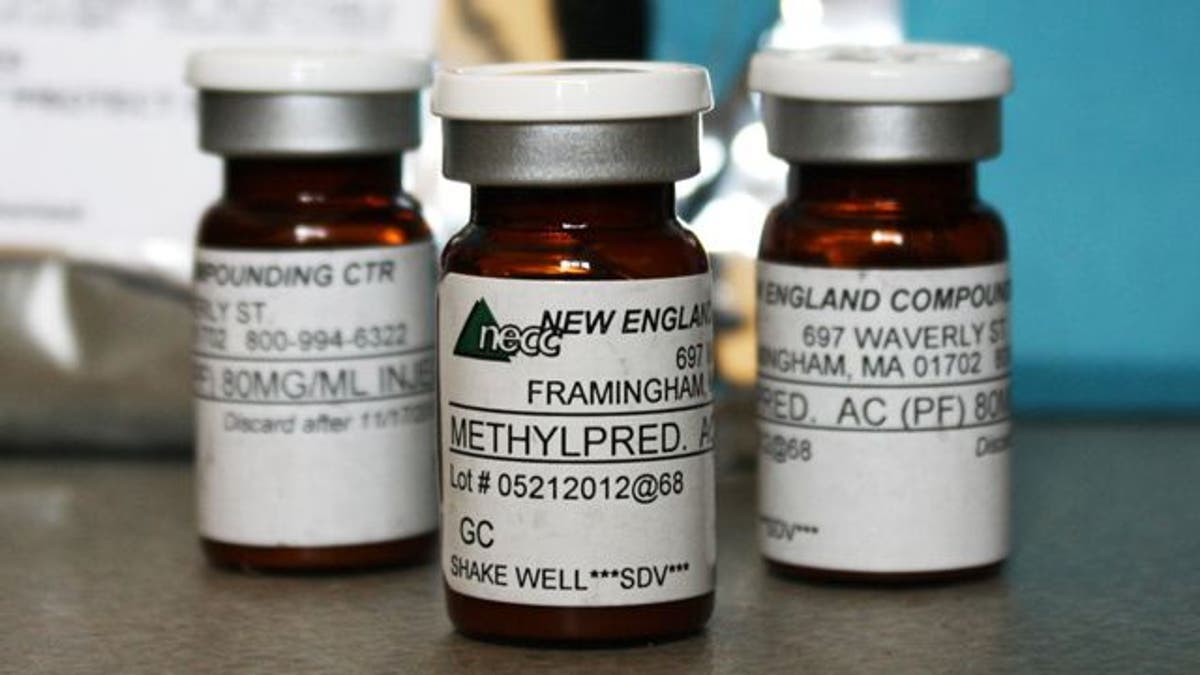
According to the Centers for Disease Control and Prevention, 39 people have died and more than 600 have been injured from injections of methylprednisolone acetate, a drug typically used to ease back pain.
New England Compounding Center, the specialty pharmacy, shut down in October after shipping tainted vials of the steroid, and filed for bankruptcy with between $1 million to $10 million in assets, court documents show.
NECC, a private company based in Framingham, Massachusetts, shipped the drug to medical facilities throughout the United States. NECC had less than $2.34 million in debts when it filed, according to the documents in U.S. Bankruptcy Court for the District of Massachusetts.
The pharmacy's equity shareholders are Carla Conigliaro with a 55 percent stake, Barry Cadden with a 17.5 percent stake, Lisa Conigliaro Cadden with a 17.5 percent stake and Gregory Conigliaro with a 10 percent stake, the documents show. In bankruptcy, the equity of a company typically has no value.
Its largest unsecured creditor is McKesson Drug and it owes it $143,169, the documents show.
The company said in a statement that it has filed papers with the court to pursue a greater, quicker payout to its creditors than they could achieve through piecemeal litigation.
NECC said Keith Lowey would be NECC's independent director and chief restructuring officer. He will oversee setting up a compensation fund.
"We want to assemble a substantial fund, and then distribute it fairly and efficiently to those who are entitled to relief," Lowey said in a statement.
NECC's bankruptcy counsel is Daniel Cohn of Murtha Cullina LLP.
Before the deadly outbreak, NECC escaped harsh punishment from health regulators several times in the years leading up to the health crisis that has raised questions about oversight of the customized drug mixing industry, Massachusetts records show.
Problems at NECC date as far back as 1999, the year after it began operations, according to hundreds of pages of documents obtained under a Freedom of Information Act request.
And the documents show regulators refraining from the harshest sanctions available to them, even as the list of complaints against NECC continued to grow.
The documents came to light after steroid shots from NECC were given to thousands of patients across the country.
Among the reported problems was a company official handing out blank prescriptions. And an outside evaluation firm found inadequate documentation and inadequate process controls involving sterilization at NECC in 2006, the documents show.
