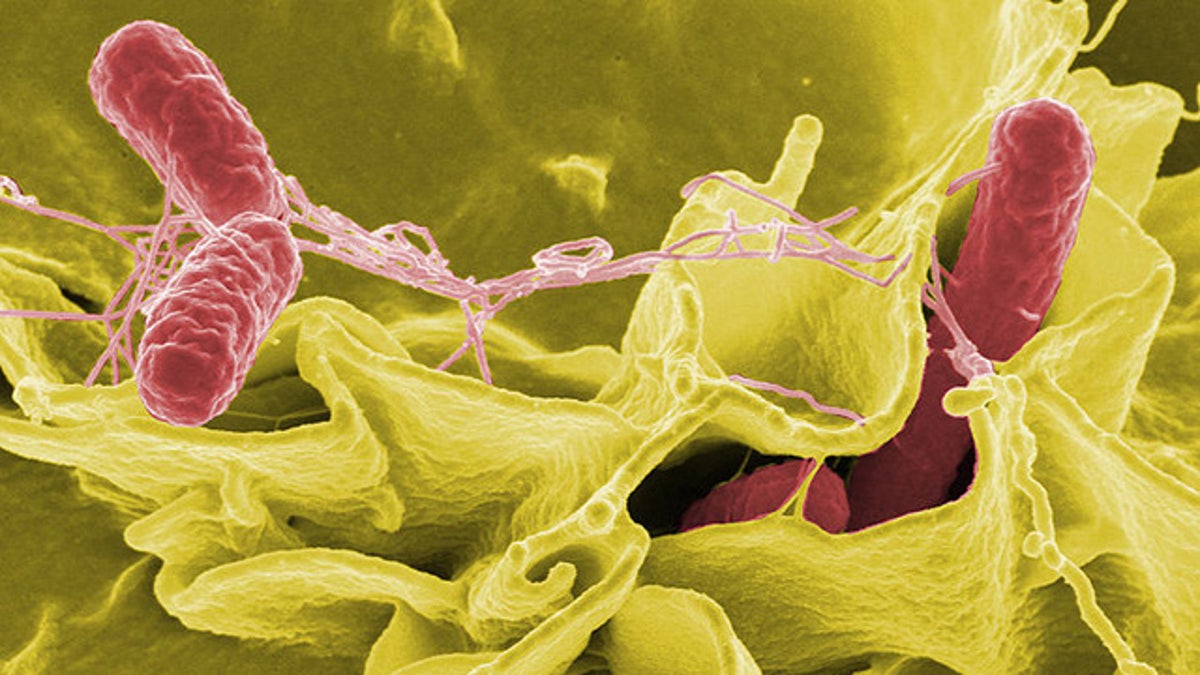
Color-enhanced scanning electron micrograph showing Salmonella typhimurium (red) invading cultured human cells. (Rocky Mountain Laboratories, NIAID, NIH)
MRSA, E. coli, salmonella – just the mention of these bacterial infections can send you running for the hand sanitizer – especially in light of the recent E. coli outbreak in Europe, which has killed at least 40 people and sickened nearly 3,500 others.
And what’s even scarier about this deadly strain of E. coli is that it’s resistant to antibiotics.
Antibiotic-resistant bacteria is nothing new. Just think about MRSA (methicillin-resistant Staphylococcus aureus), which is an infection that has been terrorizing hospitals for years. The Centers for Disease Control and Prevention said on its website that reducing this type of infection in “both health care and community settings continues to be a high priority,” and now it looks like the agency as well as other health organizations could be getting some help from researchers at the Lawrence Livermore National Laboratory in Livermore, Calif.
We spoke with Dr. Paul Jackson of the Biosciences and Biotechnology Division at the laboratory about a recent discovery that could help combat antibiotic-resistant bacteria by using the bacteria’s own genes.
Q. How exactly does this work?
A. What we’ve done is we’ve identified genes that encode proteins that are involved in different aspects of the cell’s own metabolism. So the idea is rather than go after and knocking out some critical pathway with an antibiotic, we’re going in there and basically co-opting the system and taking the genes that the organism normally has – expressed proteins encoded by those genes – and turn them around back against the pathogenic cells.
Q. How did you make this discovery?
A. We were looking for similar proteins made by bacteriophage (viruses that target bacteria) that actually will infect and kill bacterial cells, so these are viruses that kill bacterial cells… and in the process of searching for these, we were actually searching bacterial genomes because sometimes when the phage infect, their genome gets incorporated into the bacterial genome and you can go hunting for this sort of thing, and we stumbled upon these other enzymes encoded in the genome.
Q. You said at first you couldn’t figure out what they were all about – what did you end up finding out?
A. I went back and historically, I looked within E. coli to identify what the genes were identified as being important for, so back in the 1960s people would knock out a gene and find out what – without really knowing what its function was – specifically what role it had in the cell. So it very quickly became apparent that these were involved in cell wall metabolism. And then when we started looking at the function of the proteins and what they actually attacked, then it began to piece itself together and to figure out what they actually normally do.
Q. How can we incorporate these proteins in the fight against infections such as E. coli, MRSA and salmonella?
A. A group here actually has FDA approval to spray a mixture of six different bacteriophage on processed meats to knock down the listeria, so we know the process will work. We think there are two applications. The first obviously is just surfaces where you will spray it on, not on an individual scale, but on a massive scale (like a processing plant). Hypothetically, if the E. coli is on the surface, which is where it would normally be, then the spray should kill that bacterium. And remember, the protein to us is completely harmless. It has no impact whatsoever on any sort of vertebrae because it affects parts of the cell wall that are specific to bacteria. We don’t have any sorts of things like that in our cell walls.
Q. And what about the second application?
A. The other area that I’m actually more interested in is on wounds, including surgical wounds where you could after the surgery, or even if there’s just a wound, in the case of somebody who has been injured, you may be able to use these because they are proteins – they’re not going to kill the tissue. They shouldn’t have any impact at all on that human tissue at all. There are experiments left to be done, but certainly on the surface from a topical standpoint, they should very specifically knock down some of the pathogens that are the problematic pathogens- like MRSA.
Q. What do you see for the future?
A. There’s a lot of different directions one can go. You can look at decontamination, you can look at use of research and things prophylactically so that you don’t get the infections in the first place, so rather than treating somebody with an infection, you’d actually treat so they’d never get an infection. And so that’s the direction we’re thinking now.
Click here for more information about the Lawrence Livermore National Laboratory.
