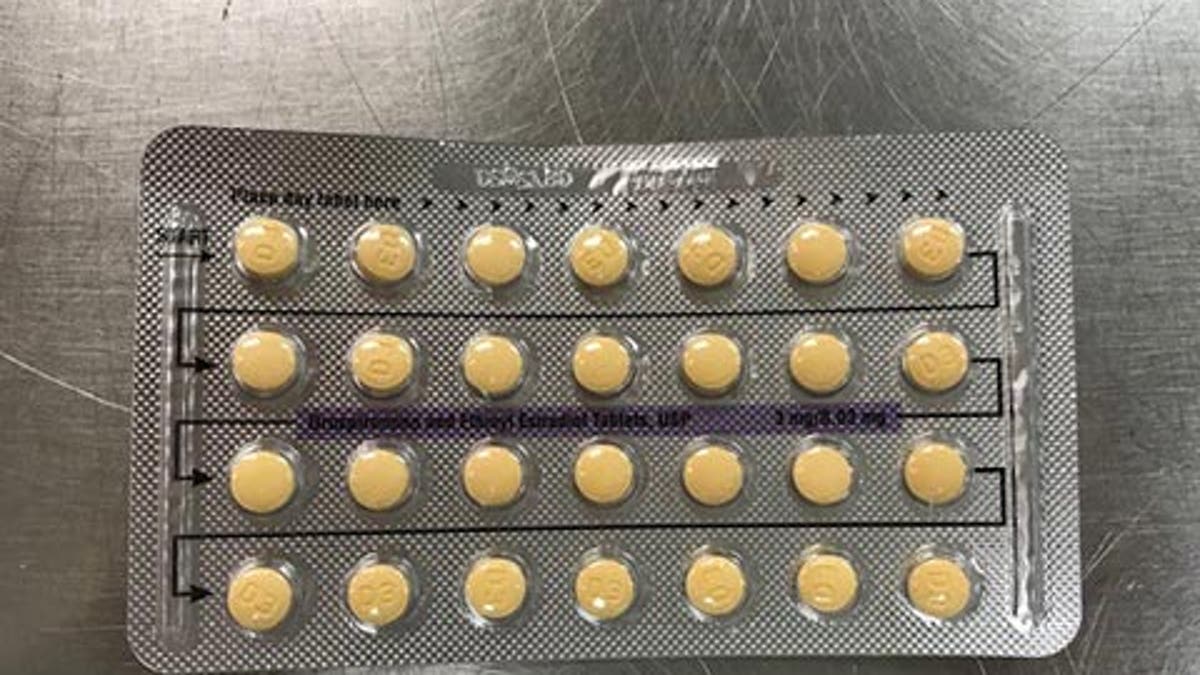
The company said a packaging error, which can affect the product's efficacy, led to the recall. (FDA)
A Florida company is voluntarily recalling four lots of its birth control pills over defective packaging, which may result in incorrect tablet arrangements or empty blister pockets, and can affect the product’s efficacy. The company, Apotex Corp., said the recall affects its Drospirenone and Ethinyl Estradiol Tablets, USP, which were shipped to distributors and wholesalers nationwide.
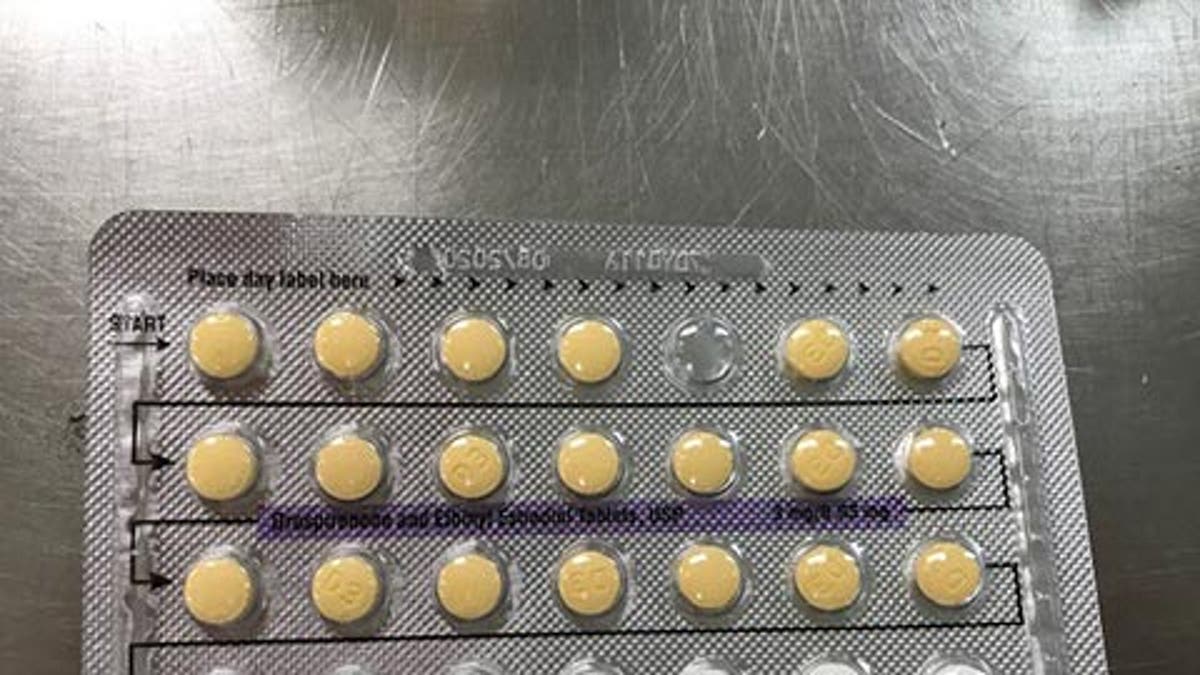
Pills may be out of order, or there may be empty blister pockets. (FDA)
“As a result of this packaging error, where a patient does not take a tablet due to a missing tablet or that a patient takes a placebo instead of an active tablet, loss of efficacy is possible due to variation in the dosage consumed,” a recall notice on the FDA website said. “To date, no case has been reported for pregnancy and adverse event to Apotex.”
30,000 POUNDS OF BEEF RECALLED OVER 'EXTRANEOUS MATERIALS'
The product, which is manufactured by Oman Pharmaceutical Products Co. LLC, under subcontract from Helm AG, Nordkanalstrasse 28 Hamburg, 20097, Germany, is available in 3MG/0.03MG, and consists of 28-film-coated, biconvex tablets. It should contain 21 yellow colored tablets, followed by 7 placebo white color tablets.

Patients are instructed to contact their health care provider for further instructions. (FDA)
The affected lots are labeled with the NDC number 60505-4183-3, and an NDC number on inner carton 60505-4183-1. The lot numbers affected include 7DY008A, 7DY009A, 7DY010A, 7DY011A, and all products have an expiration date of 8/2020.
CLICK HERE TO GET THE FOX NEWS APP
Patients who have been prescribed or received the affected medication are instructed to contact their health care provider. Those with additional questions are encouraged to contact Apotex corp. by phone-number 1-800-706-5575 (8:30am – 5:00pm, EST Monday thru Friday) or email UScustomerservice@Apotex.com .
