(REUTERS/Carlos Barria)
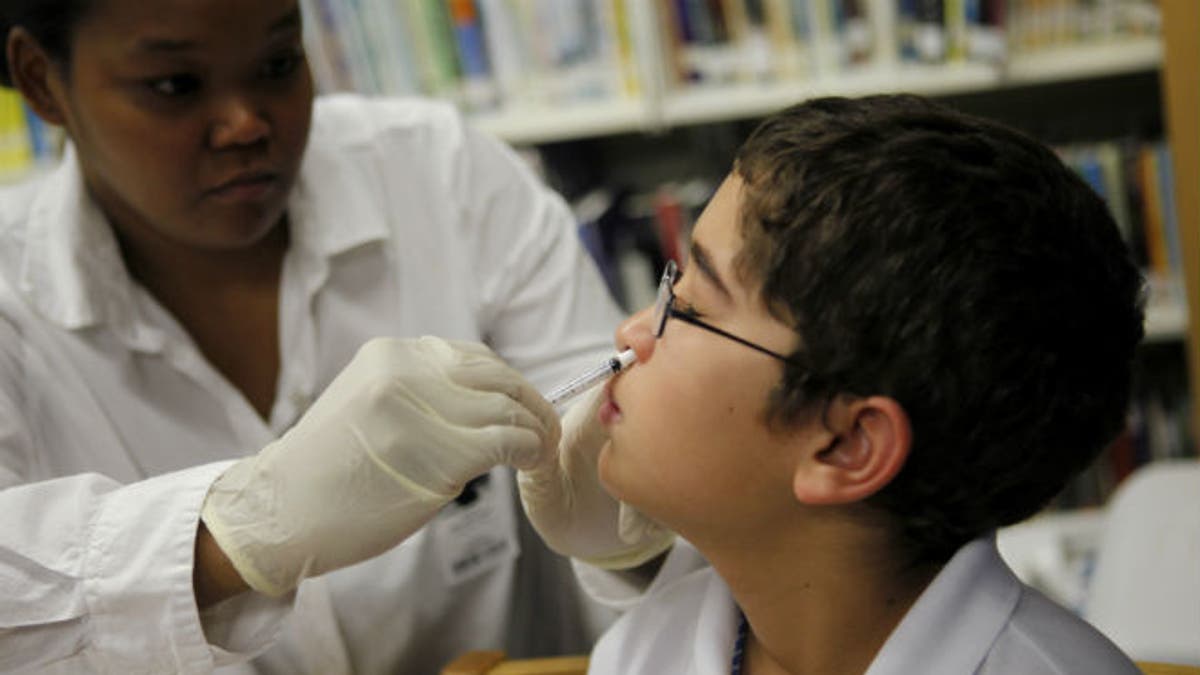
The intranasal flu vaccine, which contains live but weakened flu virus and a small amount of egg protein, does not cause adverse reactions in young people, even those with egg allergies or mild to moderate asthma, according to a new study in the U.K.
The live attenuated intranasal vaccine works well to prevent flu and eliminates the need for an injection, so in 2012 it was made the standard recommended vaccine for kids aged two to 16 years. It seems to have a similar safety profile to injected vaccines containing inactivated virus, the study team writes in the BMJ.
In the U.S., guidelines recommend the live attenuated spray or the inactivated injection, with no preference for one over the other.
But according to the Centers for Disease Control and Prevention, children younger than two, adults age 50 and older, pregnant women and people with egg allergies should not get the nasal spray, while people with asthma are warned that wheezing may increase after getting the spray. There are similar warnings in the U.K.
Based on the study results, however, lead author Dr. Paul J. Turner of Imperial College London told Reuters Health that even for kids with well-controlled asthma, the vaccine seems to be very safe.
"Most children will probably prefer a spray up the nose rather than a shot in the arm," he added.
For the new study, the researchers included 779 kids aged two to 18 years with egg allergies, which affect 2 percent to 6 percent of preschool children. More than half of the kids also had doctor-diagnosed asthma or recurrent wheeze.
The study participants were recruited from allergy centers and clinics and about a third had experienced a previous severe allergic reaction, known as anaphylaxis, to eggs in the past. Of these kids, one in five had respiratory or heart symptoms during those episodes.
The flu virus for vaccines is grown in hen's eggs, so the spray and the shot include a small amount of egg protein.
All study participants received the LAIV, most getting a total of two doses. With researchers observing them 30 minutes after vaccination and with follow-up phone calls 72 hours later, and additional follow-up one month later for certain kids, there were no reported systemic allergic reactions.
Nine kids had mild symptoms, which may have been localized allergic reactions. About 8 percent of the kids experienced lower respiratory tract symptoms in the 72-hour window, with some having parent-reported wheeze, but none were admitted to the hospital.
"In terms of egg allergies there should be no concerns at all," Turner said. "In terms of asthma, parents should consult with a person who knows the child and how well their asthma is controlled."
The spray is very similar to the injectable vaccine in terms of safety, according to Dr. Raja Rajaram, Global Medical Affairs Lead at AstraZeneca, maker of the intranasal spray vaccine.
"We do have a contraindication on the label, as do other manufacturers," but these results will prompt discussions with policymakers about changing the warnings about egg allergy and asthma, Rajaram told Reuters Health by phone.
These are good topics to discuss with your doctor when you go to get the vaccine, Turner said - and flu season proper still hasn't begun in the Northern Hemisphere of Europe, so it's still a good time to go get the spray or the shot.
