(UC San Diego School of Medicine)
Scientists have successfully replicated Alzheimer’s disease neurons with stem cells for the first time in a landmark, multi-year study – an achievement that may lead to critical new understanding of the disease, the scientists said.
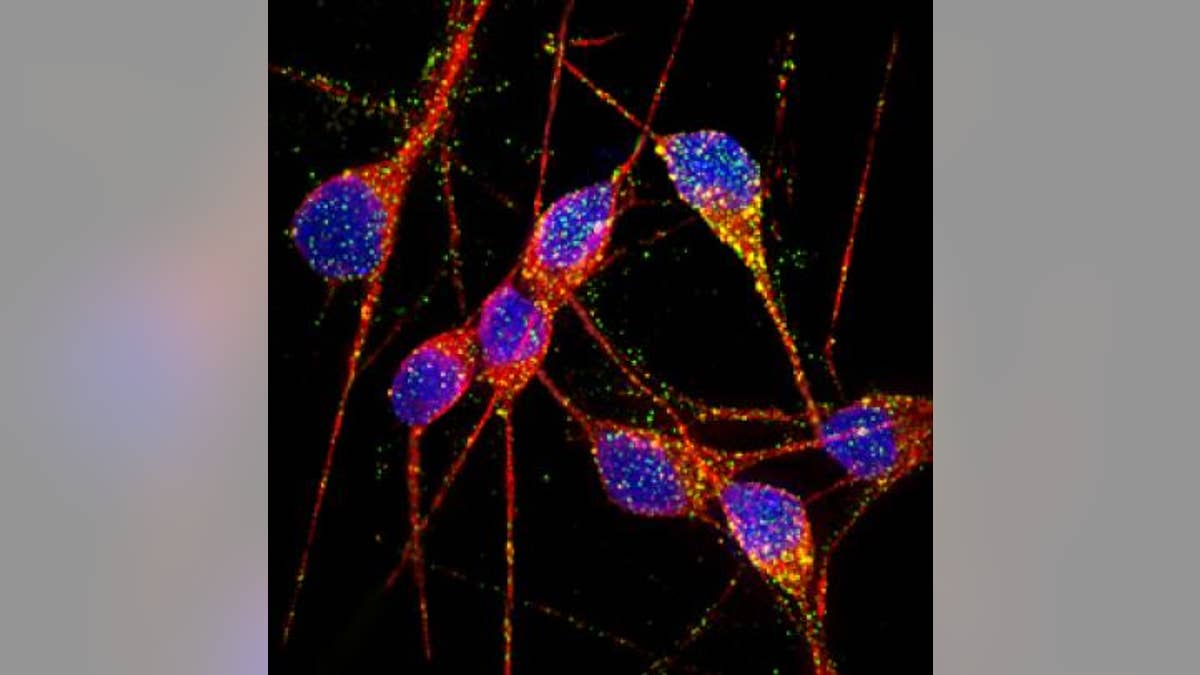
Researchers out of UC San Diego School of Medicine created in vitro models of genetic and sporadic forms of Alzheimer’s disease, using induced pluripotent stem cells (iPSC) from patients who suffered from the neurodegenerative disorder. The neurons were purified, meaning they were separated from other types of cells, to reduce variability in the experiment.
"Creating highly purified and functional human Alzheimer's neurons in a dish – this has never been done before," said senior study author Dr. Lawrence Goldstein, distinguished professor in the Department of Cellular and Molecular Medicine, Howard Hughes Medical Institute Investigator and director of the UC San Diego Stem Cell Program, in a press release.
To create the neurons, the researchers extracted fibroblasts—cells from the skin—of two patients with familial Alzheimer’s, two patients with sporadic Alzheimer’s and two people with no known neurological problems. The researchers then reprogrammed the fibroblasts into stem cells, which then differentiated into working neurons.
The iPSC-derived neurons from Alzheimer’s patients exhibited normal cell activity, formed functional synaptic contacts and – most importantly – displayed indicators of Alzheimer’s disease, such as elevated production of beta-amyloid proteins and abnormal activation of the protein kinase GSK-3.
Goldstein added the models aren’t “perfect” – they’re merely the first step. However, the research proves creating isolated Alzheimer’s neurons can be done and provides a blueprint for how to do so.
“Additional features of the model need to be developed,” Goldstein told FoxNews.com. “At this point, it’s purified neurons. So now, knowing what the purified neurons do, we want to add back defined qualities of other cells – like astrocytes. Astrocytes are normally a very important part of how neurons function, and they also play a role in resistance or susceptibility to disease.”
“We don’t know what that role is yet, but we can start to piece that back together by mixing the astrocytes back in with the neurons,” he added.
The neurons may prove to be a crucial tool for studying the causes of Alzheimer’s, as well as developing and testing drugs to treat the disease.
"We're dealing with the human brain. You can't just do a biopsy on living patients," Goldstein said. "Instead, researchers have had to work around, mimicking some aspects of the disease in non-neuronal human cells or using limited animal models. Neither approach is really satisfactory."
Using the in vitro neurons, the researchers said they could more deeply investigate the onset of Alzheimer’s disease and observe the initial processes that lead to the destruction of brain cells. Currently, dementia research is mostly centered around studies of post-mortem tissues.
“The way to think about it is, if you want to understand what goes wrong early – if you only get post-mortem tissue, a lot of the damage is already done,” Goldstein said.
“Suppose you work for the NTSB and you have to study a plane crash,” he explained. “You can get a lot of information about the crash from the wreckage, but the black box tells you what went wrong early. That’s incredibly important information for preventing crashes. We’re looking for the black box of Alzheimer’s.”
Already, the researchers said, observing the neurons has shed some light on Alzheimer’s disease onset. In particular, “we show that one of the early changes in Alzheimer's neurons thought to be an initiating event in the course of the disease turns out not to be that significant," Goldstein said.
“What we observed is, it was not the beta-amyloid fragments causing biochemical abnormalities, but it was a pre-cursor to that, called beta-CTS,” he said, adding that this finding will likely inspire a lot of debate among scientists.
According to Goldstein, the next step for using this research would be to begin testing drugs and scaling up the technology to test more patients.
“From the standpoint of drug development, here’s the core problem: we don’t have any drugs so we don’t exactly know what it’s going to take to develop them,” Goldstein said, “We think by having true human neurons to work with we can increase the speed and likelihood of finding effective drugs.”
Goldstein said continuing to research Alzheimer’s disease is critical in order to reduce the economical and emotional toll the disease takes on the nation.
“People make this interesting mistake where they say it’s just a disease of the elderly – and who cares?” Goldstein said. “The truth is, a 70 year-old person who doesn’t have the disease can be very productive economically and socially, while those who have the disease can be a drain in terms of cost of care. Projections are that the cost of Alzheimer’s will go into the trillions. It’s a real substantial impact.”
The study was published Wednesday in the online version of the journal Nature.
